Laser assisted photocatalytic reduction of metal ions by graphene oxide†
Abstract
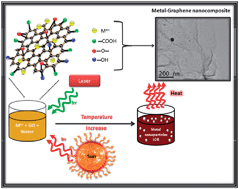
This work provides a new approach for the solution processable synthesis of metal–graphene oxide nanocomposites in aqueous solutions at ambient conditions. Using 532 nm laser or tungsten lamp irradiation of a mixture of graphene oxide (GO) and metal ions derived from HAuCl4 or AgNO3 we report the photocatalytic reduction of the metal ions simultaneously with partial

 Please wait while we load your content...
Please wait while we load your content...