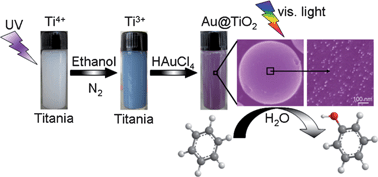
We developed a facile in situ method of preparing noble-metal plasmonic photocatalysts M@TiO2 (M = Au, Pt, Ag). In this method, the UV irradiation of TiO2 powder dispersed in absolute ethanol generates some Ti3+ ions on the surface of TiO2 particles and these Ti3+ ions, upon addition of a noble-metal salt in the dark, reduce the metal cations to deposit metal nanoparticles on the TiO2 surface. This Ti3+-ion-assisted synthesis leads to a homogeneous loading of noble-metal nanoparticles on the surface of TiO2 particles, which allows photocatalytic reactions to take place under visible-light on the whole TiO2 surface. Among the three photocatalysts M@TiO2 (M = Au, Pt, Ag), Au@TiO2 exhibits a high yield (63%) and selectivity (91%) for the oxidation of benzene to phenol in aqueous phenol. For this photocatalytic reaction, our study suggests a mechanism in which the visible-light absorption by the Au nanoparticles causes electron transfer from the Au nanoparticles to the TiO2 particle, and the electron-depleted Au oxidizes phenoxy anions to form phenoxy radicals that oxidize benzene to phenol.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...