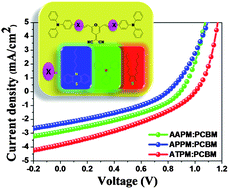
We successfully synthesized a series of novel symmetrical solution processable small molecules (APPM, AAPM and ATPM) consisting of the electron-accepting moiety (2-pyran-4-ylidenemalononitrile) (PM) and the electron-donating moiety (triphenylamine) linked by different electron-donating moieties (phenothiazine, triphenylamine and thiophene) through a Suzuki coupling reaction. Differential scanning calorimetry (DSC) measurement indicates that APPM and AAPM shows relatively high glass-transition temperature of ca. 137 °C and 163 °C, while the melting point of ATPM is at ca. 164 °C. UV-vis absorption spectra show that the combination of the PM moiety with moieties with a gradually increased electron-donating ability results in an enhanced intramolecular charge transfer (ICT) transition, which leads to an extension of the absorption spectral range and a reduction of the band gap of the molecules. Both cyclic voltammetry measurement and theoretical calculations displayed that the highest occupied molecular orbital (HOMO) energy levels of the molecules could be fine-tuned by changing the electron-donating ability of the electron-donating moieties. The bulk heterojunction (BHJ) photovoltaic devices with a structure of ITO/PEDOT/PSS/small molecules/PCBM/LiF/Al were fabricated by using the small molecules as donors and (6,6)-phenyl C61-butyric acid methyl ester (PCBM) as acceptor. Power conversion efficiencies (PCE) of 0.65%, 0.94% and 1.31% were achieved for the photovoltaic devices based on APPM/PCBM, AAPM/PCBM and ATPM/PCBM under simulated AM 1.5 illumination (100 mW cm−2), respectively. The open circuit voltage of 1.0 V obtained from the device based on ATPM/PCBM is one of the highest values for organic solar cells based on solution processable small molecules.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...