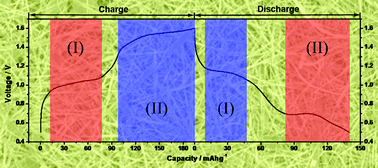
Aqueous lithium ion batteries have been widely considered as promising “green” batteries due to several advantages, such as low toxicity, low cost, high safety, as well as high ion conductivity. But unlike the great effort devoted to understanding the lithium insertion/extraction process in non-aqueous lithium ion batteries, the knowledge about this in aqueous electrolytes is still lacking research at present. In this work, taking a new anode material of single-crystalline Ag2V4O11 nanobelts as an example, we investigated the charge-discharge reaction mechanism of aqueous lithium ion batteries for the first time. A two-step reaction mechanism was proposed and it was also deduced that crystallinity loss of the electrode materials and partial irreversibility of silver oxidation are the key reasons for rapid capacity fading. We expect this work to provide a scientific platform that could help to investigate and evaluate other electrode materials in this research area.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...