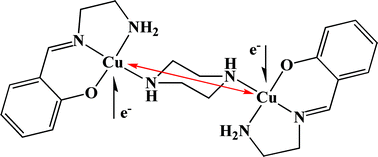
The synthesis and the characterization of new dinuclear copper(II) compounds of general formula [(La–d)2Cu2(μ-N–N)](ClO4)2 (1–6) with either neutral aliphatic diamine (N–N = piperazine, pip) or aromatic diimine (N–N = 4,4′-bipyridine, 4,4′-bipy) linker are reported. The copper ligands L− (La− = (E)-2-((2-aminoethylimino)methyl)phenolate, Lb− = (E)-2-((2-aminopropylimino)methyl)-phenolate, Lc− = (E)-2-((2-aminoethylimino)methyl)4-nitrophenolate, Ld− = (E)-2-((2-aminoethylimino)methyl)4-methoxyphenolate) are NNO tridentate Schiff bases derived from the monocondensation of a substituted salicylaldehyde 5-G-salH (G = NO2, H, OMe) with ethylenediamine, en, or 1,3-propylenediamine, tn. The crystal structures of compounds [(La)2Cu2(MeOH)2(μ-4,4′-bipy)](ClO4)2 (1·2MeOH), [(Lb)2Cu2(MeOH)2(μ-4,4′-bipy)](ClO4)2 (2·2MeOH), [(Ld)2Cu2(μ-4,4′-bipy)](ClO4)2 (4), [(La)2Cu2(μ-pip)](ClO4)2 (5) and [(Lb)2Cu2(μ-pip)](ClO4)2 (6) have been determined, revealing the preferred (e-e)-chair conformation of the bridging piperazine in compounds 5 and 6. The presence of hydrogen-bond-mediated intermolecular interactions, that involve the methanol molecules, yields dimers of dinuclear units for 1·2MeOH, and infinite zig-zag chains for 2·2MeOH. The temperature dependences of the magnetic susceptibilities χM(T) for all compounds were measured, indicating the presence of antiferromagnetic Cu–Cu exchange. For the compounds 2–4 with 4,4′-bipy, the coupling constants J are around −1 cm−1, while in compound 1 no interaction could be detected. The compounds 5 and 6 with piperazine display higher Cu–Cu magnetic interactions through the σ-bonding backbone of the bridging molecule, with J around −8 cm−1, and the coupling is favoured by the (e-e)-chair conformation of the diamine ring. The non-aromatic, but shorter, linker piperazine gives rise to stronger Cu–Cu antiferromagnetic couplings than the aromatic, but longer, 4,4′-bipyridine. In the latter case, the rotation along the C–C bond between the two pyridyl rings and the consequent non co-planarity of the two copper coordination planes play an important role in determining the magnetic communication. EPR studies reveal that the dinuclear species are not stable in solution, yielding the solvated [(L)Cu(MeOH)]+ and the mononuclear [(L)Cu(N–N)]+ species; it appears that the limited solubility of the dinuclear compounds is responsible for their isolation in the solid state.

 Please wait while we load your content...
Please wait while we load your content...