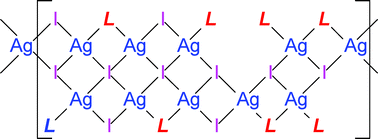
Reaction of (NH4)2[MO2S2] (M = Mo or W) with KI, CuCl and 1,3-diazepane-2-thione (Diap) in acetone affords air- and moisture-stable mixed-metal cluster compounds [MOS3(CuDiap)3]I (1 and 2). Attempts to produce [WS4Ag2(MimPh)4] (MimPh = 2-mercapto-1-phenylimidazole) led to the unexpected polymeric compound [Ag5I5(MimPh)4]n (4), subsequently obtained from a rational direct reaction between AgI and MimPh in chloroform. The complexes have been characterized by IR, 1H and 13C NMR spectroscopy, and single-crystal diffraction. 1 and 2 have crystallographic threefold rotation symmetry, with an incomplete distorted cube MS3Cu3 core bearing terminal oxo and Diap ligands on M and Cu, respectively. The cube vertex opposite M is empty, giving an overall +1 cationic cluster and a separate I− anion too distant from the three Cu atoms to be considered as covalently bonded and resulting in discrete ion pairs in the crystal structures. This arrangement is different from previously reported related OMS3(CuL)3X complexes (L = monodentate ligand, X = halide), in which X, when present, is directly bonded to one, two or three Cu atoms. 4 has a one-dimensional polymeric chain structure in which silver displays five different approximately tetrahedral coordination environments, iodide ions serve as μ2, μ3 and μ4 bridges, and the thione ligands are each either terminal or bridging. This unusually complex structure for a relatively simple chemical formula represents only the fifth example of a complex (AgI)nLm in which L is a neutral S-donor ligand, and the five structures display a wide range of individual features. In all three of the new structures, N–H⋯S and/or N–H⋯I hydrogen bonds are found.

 Please wait while we load your content...
Please wait while we load your content...