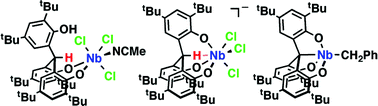
The synthesis and reactivity of niobium complexes incorporating a tripodal triphenol (tris(3,5-tert-butyl-2-hydroxylphenyl)methane = H3[O3]) have been investigated. Addition of one equivalent of NbCl5 in CH3CN to H3[O3] in toluene led to partial HCl elimination, giving [H(O3)]NbCl3(CH3CN) (1) with a bidendtate bis(aryloxide) ligand and a pendant phenol arm. Treatment of 1 with THF afforded [H(O3)]NbCl3(THF) (2). Deprotonation of 1 with NEt3 in toluene promoted coordination of the pendant phenol group to generate (Et3NH)[(syn-O3)NbCl3] (3-syn). Prolonged heating of 3-syn resulted in clean conversion to the anti isomer (3-anti). Attempted deprotonation of 2 with PhCH2MgCl provided [H(O3)]Nb(CH2Ph)3 (4), in which alkylation took place at the metal center but the pendant phenol arm remained intact. When 3-syn was treated with PhCH2MgCl, [O3C]Nb(CH2Ph) (5) was produced via C–H activation of the methine C–H bond. The analogous reaction with 3-anti provided a benzylidene complex [anti-O3]Nb(CHPh)(THF) (6). During the course of the reaction, the anti ligand conformation is retained. Upon heating, 4 underwent methine C–H and phenol O–H activation, yielding the metalatrane 5. Complexes 1, 3-syn, 3-anti, 4, and 5 were characterized by X-ray diffraction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...