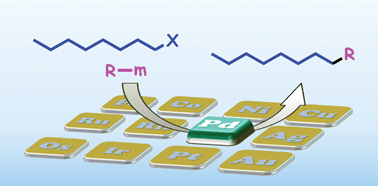
Cross-coupling reactions have become indispensable tools for creating carbon–carbon (or heteroatom) bonds in organic synthesis. Like in other important transition metal catalyzed reactions, such as metathesis, addition, and polymerization, unsaturated compounds are usually employed as substrates for cross-coupling reactions. However during the past decade, a great deal of effort has been devoted to the use of alkyl halides as saturated compounds in cross-coupling reactions, which has resulted in significant progress in this undeveloped area by introducing new effective ligands. Many useful catalytic systems are now available for synthetic transformations based on Csp3–Csp3, Csp3–Csp2 and Csp3–Csp bond formation as complementary methods to conventional Csp2–Csp2, Csp2–Csp and Csp–Csp coupling. This tutorial review summarizes recent advances in cross-coupling reactions of alkyl halides and pseudohalides catalyzed by a palladium complex.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...