On the stability of the elusive HO3 radical
Abstract
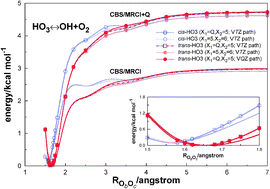
The dissociation of HO3 into OH + O2 has been studied in a systematic and consistent way using the multireference configuration interaction method. Upon extrapolation of the calculated raw energies to the complete basis set limit and using jointly with a recent realistic estimate of the zero-point vibrational energy, the energy for OO–OH bond-breaking in the trans isomer is predicted to be of D0 = (2.4 ± 0.1) kcal mol−1, where the uncertainty reflects only the one inherent to the extrapolation. The average value so obtained falls short of the commonly accepted experimental counterpart by 0.5 kcal mol−1. Reasons for the deviation are advanced, as well as an estimate of the binding energy for the cis-HO3 isomer which is predicted to have a somewhat smaller binding energy than trans-HO3, but likewise the latter dissociates without a barrier to the same products.

 Please wait while we load your content...
Please wait while we load your content...