The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates
Abstract
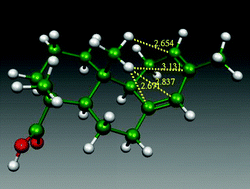
The CH/π hydrogen bond is an attractive molecular force occurring between a soft acid and a soft base. Contribution from the dispersion energy is important in typical cases where aliphatic or aromatic CH groups are involved. Coulombic energy is of minor importance as compared to the other weak hydrogen bonds. The hydrogen bond nature of this force, however, has been confirmed by AIM analyses. The dual characteristic of the CH/π hydrogen bond is the basis for ubiquitous existence of this force in various fields of chemistry. A salient feature is that the CH/π hydrogen bond works cooperatively. Another significant point is that it works in nonpolar as well as polar,
- This article is part of the themed collection: Weak hydrogen bonds – strong effects?
 Please wait while we load your content...
Please wait while we load your content...