Effects of external electric fields on double proton transfer kinetics in the formic acid dimer
Abstract
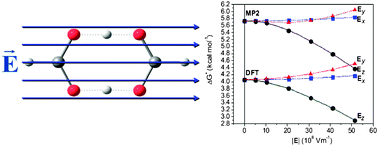
Molecules can be exposed to strong local electric fields of the order of 108–1010 V m−1 in the biological milieu. The effects of such fields on the rate constant (k) of a model reaction, the double-proton transfer reaction in the formic acid dimer (FAD), are investigated. The barrier heights and shapes are calculated in the absence and presence of several static homogenous external fields ranging from 5.14 × 108 to 5.14 × 109 V m−1 using density functional theory (DFT/B3LYP) and second order Møller–Plesset perturbation theory (MP2) in conjunction with the 6-311++G(d,p) Pople basis set. Conventional transition state theory (CTST) followed by Wigner tunneling correction is then applied to estimate the rate constants at 25 °C. It is found that electric fields parallel to the long axis of the dimer (the line joining the two carbon atoms) lower the uncorrected barrier height, and hence increase the raw k. These fields also flatten the potential energy surface near the transition state region and, hence, decrease the multiplicative tunneling correction factor. The net result of these two opposing effects is that fields increase k(corrected) by a factor of ca. 3–4 (DFT–MP2, respectively) compared to the field-free k. Field strengths of ∼3 × 109 V m−1 are found to be sufficient to double the tunneling-corrected double

 Please wait while we load your content...
Please wait while we load your content...