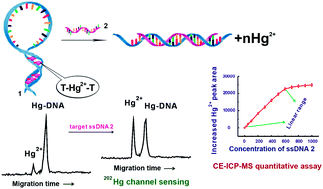
Detecting a specific DNA sequence and discriminating single base-mismatch is critical to clinical diagnosis, paternity testing, forensic sciences, food and drug industry, pathology, genetics, environmental monitoring, and anti-bioterrorism. To this end, capillary electrophoresis (CE) coupled with the inductively coupled plasma mass spectrometry (ICP-MS) method is developed using the displacing interaction between the target ssDNA and the competitor Hg2+ for the first time. The thymine-rich capture ssDNA 1 is interacted with the competitor Hg2+, forming an assembled complex in a hairpin-structure between the thymine bases arrangement at both sides of the capture ssDNA 1. In the presence of a target ssDNA with stronger affinity than that of the competitor Hg2+, the energetically favorable hybridization between capture ssDNA 1 and the target ssDNA destroys the hairpin-structure and releases the competitor as free Hg2+, which was then read out and accurately quantified by CE-ICP-MS assay. Under the optimal CE separation conditions, free Hg2+ ions and its capture ssDNA 1 adduct were baseline separated and detected on-line by ICP-MS; the increased peak intensity of free Hg2+ against the concentration of perfectly complementary target ssDNA was linear over the concentration range of 30–600 nmol L−1 with a limit of detection of 8 nmol L−1 (3s, n = 11) in the pre-incubated mixture containing 1 μmol L−1Hg2+ and 0.2 μmol L−1 capture ssDNA 1. This new assay method is simple in design since any target ssDNA binding can in principle result in free Hg2+ release by 6-fold Hg2+ signal amplification, avoiding oligonucleotide labeling or assistance by excess signal transducer and signal reporter to read out the target. Due to element-specific detection of ICP-MS in our assay procedure, the interference from the autofluorescence of substrata was eliminated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...