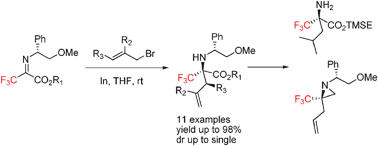
Highly diastereoselective synthesis of quaternary α-trifluoromethyl α-amino acids from chiral imines of trifluoropyruvate†‡
Abstract
An efficient method for highly diastereoselective synthesis of quaternary α-trifluoromethyl α-amino acids was developed via
- This article is part of the themed collection: Fluorine Chemistry

 Please wait while we load your content...
Please wait while we load your content...