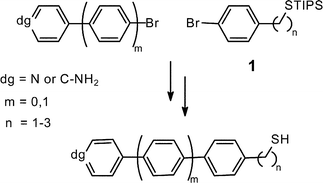
Araliphatic thiols are key molecules for the formation of self-assembled monolayers with high long-range order. If these monolayers shall act as bases for the attachment of other molecules, the respective thiols need to carry suitable functional groups, such as the amino or the pyridine group. Due to their Lewis-basicity, these groups are not compatible with the thiol group under most reaction conditions. Here, an entry into this versatile class of compounds is presented, by using fundamental building blocks in which the thiol groups are protected as triisopropylsilyl sulfides making them compatible with many reagents including Grignard reagents and palladium catalysts. With this strategy at hand, six thiols with bi- and terphenyl backbones, one to three methylene groups, and amino or pyridine head groups became accessible in short reaction sequences.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...