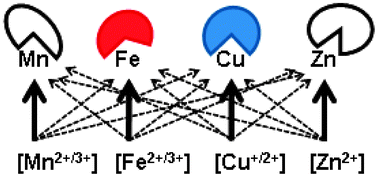
Metalloproteomics includes approaches that address the expression of metalloproteins and their changes in biological time and space. Metalloproteomes are investigated by a combination of approaches. Experimental approaches include structural genomics, which provides insights into the architecture of metal-binding sites in metalloproteins and establishes ligand signatures from the types and spacings of the metal ligands in the protein sequence. Theoretical approaches employ these ligand signatures as templates for homology searches in sequence databases. In this way, the number of metalloproteins in the iron, copper, and zinc metalloproteomes in various phyla of life has been estimated. Yet, manganese metalloproteomes remain poorly defined. Metals have catalytic and structural functions in proteins. However, additional functions have evolved. Proteins that control metal homeostasis and proteins that are metal-regulated bind metal ions transiently and are generally not accounted for in estimates from bioinformatics. Thus, metalloproteomes are dynamic and likely to be larger than present estimates suggest. This account discusses the assignment of transition metals in metalloproteins and the ensuing issues facing analytical chemists and structural and computational biologists. Biological and chemical selectivities render metal selection by metalloproteins either more stringent or less stringent depending on the metal homeostatic system of the organism, the subcellular location of the protein, and environmental factors. Failure to recognize the principles of metal utilization has led to assigning the wrong metal in metalloproteins and has missed some of the regulatory functions of transition metal ions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...