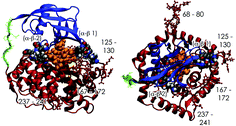
As part of ongoing research into the use of vitamin B12 (B12; cobalamin; Cbl)-based bioconjugate approaches for the oral delivery of peptides/proteins, a molecular dynamics (MD) study of the binding of a cyanocobalamin–insulin (CN–Cbl–insulin) conjugate to human transcobalamin(II) (TCII) was recently reported that provides a qualitative picture of how the human insulin protein in its open “T-state” geometry affects CN–Cbl binding to TCII. This initial analysis revealed that the B22–B30 segment of the insulin B-chain acts as a long tether that connects the larger combined insulin A/B region to CN–Cbl when this conjugation is performed at the CN–Cbl ribose 5′-hydroxy position. The experimental support for this model of the binding interaction is provided by the consequences of the successful delivery of the CN–Cbl–insulin conjugate in the production of significantly decreased blood glucose levels in diabetic STZ-rat models. In efforts to provide a more detailed description of the (CN–Cbl)–TCII complex for modeling Cbl-based bioconjugate designs, the (CN–Cbl)–TCII system and a CN–Cbl conjugate incorporating a flexible tether composed of only the B22–B30 segment of human insulin have been examined by MD simulations. The implications of these simulations are discussed in terms of successful conjugate positioning on Cbl, especially when such sites are not apparent from the diffraction studies alone, and the possibilities, as yet not reported, for dual-tethered Cbl bioconjugates for multi-component drug delivery applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...