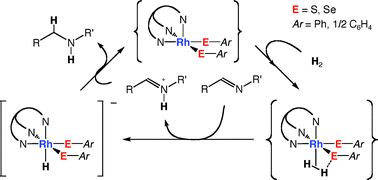
Thiolato complexes of Rh(III) bearing a hydrotris(3,5-dimethylpyrazolyl)borato ligand (TpMe2) have been prepared, and their reactivity toward H2 has been investigated. The bis(thiolato) complex [TpMe2Rh(SPh)2(MeCN)] (1) reacted with 1 atm H2 at 20 °C to produce the hydrido-thiolato complex [TpMe2RhH(SPh)(MeCN)] (2) and PhSH via heterolytic cleavage of H2. This process is reversible and in equilibrium in THF and benzene. The bis(selenolato) complex [TpMe2Rh(SePh)2(MeCN)] (4) was also converted to [TpMe2RhH(SePh)(MeCN)] and PhSeH under 1 atm H2, but the equilibrium largely shifted to 4. Reaction of the dithiolato complex [TpMe2Rh(bdt)(MeCN)] (3; bdt = 1,2-C6H4S2) with H2 occurred in the presence of amine, giving the anionic hydrido complex [TpMe2RhH(bdt)]− and an equimolar amount of ammonium cations. Catalytic activity for hydrogenation has been examined under 1 atm H2 at 20–50 °C. While 1, 2, and 4 slowly hydrogenated styrene at similar rates at 50 °C, activities for the hydrogenation of N-benzylideneaniline increased in the order, 2 < 1 < 4. Complex 3 was found to be the most active and selective catalyst for hydrogenation of imines, and thus a variety of imines were reduced at 20 °C under 1 atm H2, with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the substrate molecules completely preserved. An ionic mechanism was involved to explain such high chemoselectivity.
O bonds in the substrate molecules completely preserved. An ionic mechanism was involved to explain such high chemoselectivity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the substrate molecules completely preserved. An ionic mechanism was involved to explain such high chemoselectivity.
O bonds in the substrate molecules completely preserved. An ionic mechanism was involved to explain such high chemoselectivity.

 Please wait while we load your content...
Please wait while we load your content...