Molecular balances for quantifying non-covalent interactions
Abstract
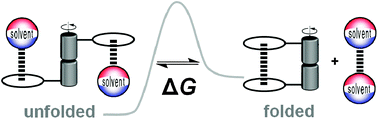
Molecular interactions underlie the whole of chemistry and biology. This tutorial review illustrates the use of rotameric folding molecules, topoisomers, atropoisomers, and tautomers as molecular balances for quantifying non-covalent interactions. This intramolecular approach enables a wide variety of interactions to be examined with a degree of geometric control that is difficult to achieve in supramolecular complexes. Synthetic variation of molecular balances allows the fundamental physicochemical origins of molecular recognition to be systematically examined by providing insights into the interplay of geometry and solvation on non-covalent interactions.

 Please wait while we load your content...
Please wait while we load your content...