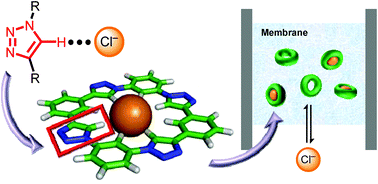
The supramolecular chemistry of anions provides a means to sense and manipulate anions in their many chemical and biological roles. For this purpose, Click chemistry facilitated the synthetic creation of new receptors and thus, an opportunity to aid in the recent re-examination of CH⋯anion hydrogen bonding. This tutorial review will focus on the privileged C–H hydrogen bond donor of the 1,2,3-triazole ring systems as elucidated from anion-binding studies with macrocyclic triazolophanes and other receptors. Triazolophanes are shape-persistent and planar macrocycles that direct four triazole and four phenylene CH groups into a 3.7 Å cavity. They display strong (log K(Cl−) = 7), size-dependent halide binding (Cl− > Br− ≫ F− ≫ I−) and a rich set of binding equilibria. For instance, the too large iodide (4.4 Å) can be sandwiched between two pyridyl-based triazolophanes with extreme positive cooperativity. Computational studies verify the triazole's hydrogen bond strength indicating it approaches the traditional NH donors from pyrrole. These examples, those of transport, sensing (e.g., ion-selective electrodes), templation, and versatile synthesis herald the use of triazoles in anion-receptor chemistry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...