Using high resolution solid state 15N and 13C NMR spectroscopy we have studied the effects of successive hydration on the 15N labeled side chain amino groups of solid poly-L-lysine (PLL) in the presence of acids. Generally, hydration leads to the formation of local “ionic fluid” phases composed by flexible side chain ammonium groups, acid anions and small amounts of water. The associated local dynamics reduces the widths of the inhomogeneously broadened 15N amino signals found for the dry states. The hydration of free base PLL—which consists of mixtures of α-helices and β-pleated sheets—is monitored by a small low-field shift of the amino group signal arising from hydrogen bonding with water, reaching eventually the value of PLL in water at pH 13. No difference for the two conformations is observed. PLL×HF adopts a similar secondary structure with isolated NHF hydrogen bonds; hydration leads only to small low-field shifts which are nevertheless compatible with the formation of ammonium groups in aqueous solution. PLL doped with small amounts of HCl contains ammonium groups which are internally solvated by neighboring free amino groups. Both nitrogen environments are characterized by different chemical shifts. Hydration with less than one water molecule per amino group leads already to a chemical shift averaging arising from fast proton motions along NHN-hydrogen bonds and fast side chain and anion motions.
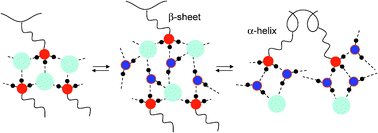
By contrast, the hydration of fully doped PLL×HBr and PLL×HCl is more complex. These systems exist only in β-pleated sheet conformations forming alkyl ammonium salt structures. Separate 15N signal components are observed for (i) the dry states, for (ii) wet β-pleated sheets and for (iii) wet α-helices which are successively formed upon hydration. Exchange between these environments is slow, but water motions lead to averaged amino group signals within each of the two wet environments. These results indicate that the different environments form domains. As the replacement of NHBr or of NHCl hydrogen bonds by NHO hydrogen bonds leads to high-field shifts the observation of separated signals is the result of different water content in the three domains. In agreement with previous X-ray powder diffraction studies we observe a dominance of the α-helical regions at above 3 water molecules per amino group in the case of PLL×HBr and at about 5 water molecules in the case of PLL×HCl, an effect arising from the limited space between β-pleated sheets and the larger volume of bromide as compared to chloride.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...