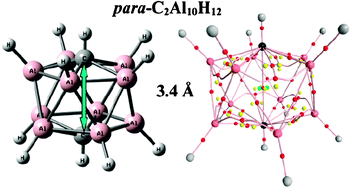
We report on the electronic structure of the 12-vertex icosahedral clusters r-X2Z10H12 and Z12H122–, where X = {C, Si} and Z = {B, Al}. The least stable cluster—with the lowest HOMO–LUMO gap (Eg)—corresponds to the ortho-X2Z10H12 isomers for all values of X = {C, Si} and Z = {B, Al}. The well-known energetic order E(para) < E(meta) < E(ortho) for r-carboranes is also valid for all compounds except r-C2Al10H12. Substitution of two atoms of carbon or silicon into the icosahedral cage B12H122− enhances considerably the stability of the system as analyzed from Eg gaps, as opposite to Al12H122−, where similar gaps are found upon double carbon or silicon substitution regardless of the positions in the cage. In order to highlight similarities and differences in the title clusters, topological analysis of the electron density was performed, together with analysis of the deviation from polyhedron icosahedral form with (i) volumes, skewness and kurtosis calculations; and (ii) continuous shape measures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...