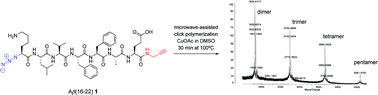
We report on the design, synthesis, and structural analysis of cyclic oligomers with an amyloidogenic peptide sequence as the repeating unit to obtain novel self-assembling bionanomaterials. The peptide was derived from the Alzheimer Aβ(16–22) sequence since its strong tendency to form antiparallel β-sheets ensured the formation of intermolecular hydrogen bridges on which the supramolecular assembly of the individual cyclic oligomers was based. The synthesis of the cyclic oligomers was performed via a microwave-assisted Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction of azido-Lys-Leu-Val-Phe-Phe-Ala-Glu-propargyl amide as the monomer. The formation of cyclic oligomers, up to pentamers (35 amino acid residues), was verified by MALDI-TOF analysis and the individual cyclic monomer and dimer could be isolated by HPLC. Gelation behavior and the self-assembly of the linear monomer and the cyclic monomer and dimer were studied by TEM, FTIR and CD. Significant differences were observed in the morphology of the supramolecular aggregates of these three peptides that could be explained by alterations of the hydrogen bond network.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...