Systems chemistry and Parrondo’s paradox: computational models of thermal cycling
Abstract
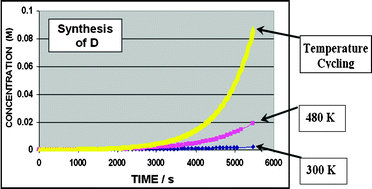
A mathematical concept known as Parrondo’s paradox motivated the development of several novel computational models of chemical systems, in which thermal cycling was explored. In these kinetics systems, we compared the rates of formation of products under temperature-cycling and steady-state conditions. We found model chemical systems that counter-intuitively predicted a greater concentration of product under oscillating temperature conditions than under fixed conditions. At a practical level, these computational models of thermal cycling suggest new applications in chemistry, biochemistry and chemical engineering. More fundamentally, these models contribute to a growing understanding that even simple chemical systems may behave paradoxically, and that forced oscillating conditions may induce such an outcome.

 Please wait while we load your content...
Please wait while we load your content...