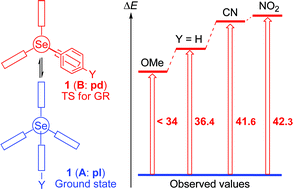
9-(Arylselanyl)triptycenes (1: p-YC6H4SeTpc) should supply the planar structure (pl) around the p-YC6H4Se (ArSe) group in the ground state, irrespective of the p-Y substituents. 1 with Y = H (a), NMe2 (b), OMe (c), Cl (d), CN (e) and NO2 (f) are prepared. Structures of 1a–d and 1f are determined by X-ray analysis and dynamic 1H NMR spectroscopy is applied to 1a, 1c, 1e and 1f. For convenience of discussion, the structure of 1 is defined as follows: a conformer around the triptycyl group in 1 is called A where the Se–CAr bond is placed in the bisected area between two phenyl planes of the triptycyl group and it is B where the bond is on a phenyl plane of the triptycyl group. A conformer for the Ar group is named pl where the Se–CTpc bond is on the Ar plane, while it is pd if the bond is perpendicular to the plane. The structure of 1 is confirmed to be (A: pl) in the ground state by X-ray analysis. 1 (A: pl) changes to the equivalent one via a transition state of 1 (B: pd) (the gear process). The activation energies are determined by dynamic 1H NMR spectroscopy: the values are 36.4, 41.6, 42.3 and <34 kJ mol−1 for 1a, 1e, 1f and 1c, respectively. The MP2 level of calculations reproduced the observed values: they are evaluated to be 34, 39, 40 and 29 kJ mol−1 for 1a, 1e, 1f and 1b′ (Y = NH2), respectively, where 1b′ is employed in place of 1c. Another process (the isolated rotation process) is also operating for the interconversion of 1 (A: pl), which proceeds via1 (A: pd). The activation energies for the process are predicted to be 25, 30, 30 and 20 kJ mol−1 for 1a, 1e, 1f and 1b′, respectively, at the MP2 level. The results demonstrate that (A: pl) is the global minimum in 1: the 1 (A: pl) structure is well established for all Y examined in the ground state.

 Please wait while we load your content...
Please wait while we load your content...