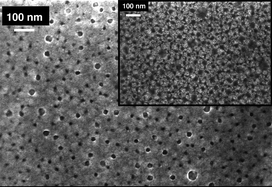
The goal of this work has been to fabricate very thin, highly porous Ir films, of use in a wide variety of applications. To achieve this objective, Ir was electrodeposited on a Au-coated quartz crystal, allowing in situ mass measurements to be carried out while concurrently monitoring the charge passed, as a function of the deposition potential, H2IrCl6 concentration, and time. Ir thin films (<5 nm) could be deposited at 100% charge efficiency at an optimum potential of 0.1–0.2 V vs. RHE, all in room temperature 0.5 M H2SO4 containing various concentrations of H2IrCl6. Field emission scanning electron microscopy and in situ surface area measurements showed that the Ir films are uniform and yet highly porous (up to 50%), independent of film thickness. We were also able to efficiently convert the electrodeposited Ir to Ir oxide, without significant loss of metal or diminished coating adhesion, while also maintaining their very high surface area properties. These interesting thin films are very promising for a wide variety of electrocatalytic and sensing applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...