The luminescence properties of the three new stoichiometrically mixed lanthanide complexes, Sm1/2Eu1/2(PBI)3·bpy·H2O (1), Sm1/2Tb1/2(PBI)3·bpy·H2O (2) and Eu1/2Tb1/2(PBI)3·bpy·H2O (3) [HPBI = 3-phenyl-4-benzoyl-5-isoxazolone; bpy = 2,2′-bipyridine] have been compared with those of the analogous single lanthanide ion systems, Sm(PBI)3·bpy·H2O (4), Eu(PBI)3·bpy·H2O (5) and Tb(PBI)3·bpy·H2O (6). Compound 5 was structurally characterized by single-crystal X-ray diffraction, and crystallizes in the triclinic space groupP![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a = 12.839(3) Å, b = 13.863(3) Å, c = 16.379(3) Å, α = 81.66(3)°, β = 73.32(3)°, γ = 89.26(3)° and V = 2762.0(10) Å3. The crystal structure of 5 comprises an assembly of mononuclear species, each of which features a central Eu3+ cation coordinated to three bidentate PBI ligands, a bidentate bipy ligand, and a water molecule. The overall geometry of the nonacoordinate array is that of a distorted monocapped trigonal prism. The X-ray diffraction study of 5 also revealed many interesting π–π, interplanar and intermolecular hydrogen-bonding interactions. The mixed lanthanide complexes 1–3 exhibit interesting dual emissions in the visible region. The quantum yields and lifetime measurements for 1–3 support the premise that Ln → Ln energy transfer occurs in these mixed lanthanide systems, along with the usual ligand-to-metal triplet energy pathways.
with a = 12.839(3) Å, b = 13.863(3) Å, c = 16.379(3) Å, α = 81.66(3)°, β = 73.32(3)°, γ = 89.26(3)° and V = 2762.0(10) Å3. The crystal structure of 5 comprises an assembly of mononuclear species, each of which features a central Eu3+ cation coordinated to three bidentate PBI ligands, a bidentate bipy ligand, and a water molecule. The overall geometry of the nonacoordinate array is that of a distorted monocapped trigonal prism. The X-ray diffraction study of 5 also revealed many interesting π–π, interplanar and intermolecular hydrogen-bonding interactions. The mixed lanthanide complexes 1–3 exhibit interesting dual emissions in the visible region. The quantum yields and lifetime measurements for 1–3 support the premise that Ln → Ln energy transfer occurs in these mixed lanthanide systems, along with the usual ligand-to-metal triplet energy pathways.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a = 12.839(3) Å, b = 13.863(3) Å, c = 16.379(3) Å, α = 81.66(3)°, β = 73.32(3)°, γ = 89.26(3)° and V = 2762.0(10) Å3. The crystal structure of 5 comprises an assembly of mononuclear species, each of which features a central Eu3+
with a = 12.839(3) Å, b = 13.863(3) Å, c = 16.379(3) Å, α = 81.66(3)°, β = 73.32(3)°, γ = 89.26(3)° and V = 2762.0(10) Å3. The crystal structure of 5 comprises an assembly of mononuclear species, each of which features a central Eu3+ 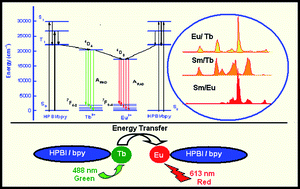

 Please wait while we load your content...
Please wait while we load your content...