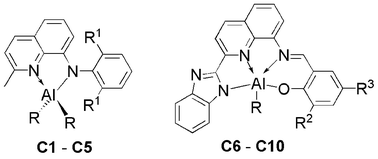
Treatment of N-aryl-2-methylquinolin-8-amines (L1–L3) with one equivalent AlMe3 or AlEt3 afforded dialkyl aluminum compounds (C1–C5), whereas the stoichiometric reaction of 2-((2-(1H-benzo[d]imidazol-2-yl)quinolin-8-ylimino)methyl)phenols (L4–L6) with either AlMe3 or AlEt3 produced monoalkyl aluminum compounds (C6–C10). All the organoaluminum compounds were characterized by 1H, 13C NMR and elemental analysis, and the molecular structures of representative compounds were confirmed by X-ray crystallography. With bidentate ligands, compounds C1 and C3 showed tetrahedron geometry around Al center, while compound C7 has a distorted square pyramidal geometry around Al center with the framework comprising the tetradentate ligand. The dialkyl aluminum compounds (C1–C5) performed high catalytic activities towards the ring opening polymerization (ROP) of ε-caprolactone (ε-CL), in parallel, the monoalkyl aluminum compounds (C6–C10) showed negative results for the polymerization of ε-CL. In the presence or absence of benzyl alcohol (BnOH), the four-coordination aluminum compounds (C1–C5) are both highly active towards the ring opening polymerization (ROP) of ε-caprolactone with resulting high conversation of ε-caprolactone and polymers with high molecular weight. In the presence of one equivalent of BnOH, polymerization of ε-caprolactone proceeded in a living manner and molecular weights of the obtained poly(ε-caprolactone)s could be precisely controlled by adapting the reaction conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...