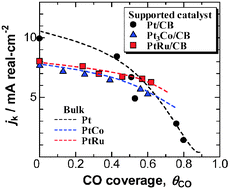
The temperature-dependence of the hydrogen oxidation reaction (HOR) rate was examined at commercial Pt, Pt3Co, PtRu, and PtRu1.5 nano-sized catalysts (diameter, d = ca. 3 nm) supported on carbon black in 0.1 M HClO4 solution in the presence and absence of carbon monoxide by use of a channel flow electrode at temperatures from 30 to 90 °C. It was found that the values of the apparent rate constant kapp (per real Pt active surface area) for the HOR at these supported catalysts agreed beautifully with those of the corresponding bulk electrodes in the whole temperature range. The dependence of the kinetically controlled current density (jk) on CO coverage at each supported catalyst was also identical to that of the bulk. Hence, no particle size effect was observed on the HOR activity and the CO tolerance, at least, was brought down to d = 3 nm.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...