Imido transfer of sulfonylimino-λ3-bromane makes possible the synthesis of sulfonylimino-λ3-iodanes†
Abstract
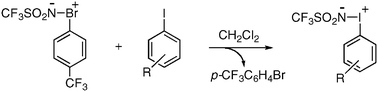
Sulfonylimino-λ3-bromane functions as a reactive nitrenoid, because of the hyperleaving group ability of aryl-λ3-bromanyl groups, and undergoes transimidations to iodobenzenes at room temperature under metal-free conditions probably via an SN2-type nitrenoid transition state, yielding sulfonylimino-λ3-iodanes.

 Please wait while we load your content...
Please wait while we load your content...