Synthesis and properties of fluorene or carbazole-based and dicyanovinyl-capped n-type organic semiconductors†‡
Abstract
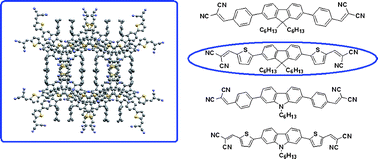
Dicyanovinyl-substituted compounds as electron transport materials are rarely studied, especially oligomers. A new series of fluorene or carbazole-based and dicyanovinyl-capped oligomers consisting of benzene or thiophene segments were synthesized using the Stille coupling reaction and their physical properties were investigated. Optical spectra show that the introduction of electron-accepting groups induces an intramolecular charge transfer, resulting in a shift of the absorption onset toward longer wavelengths. Moreover, the optical spectra of their films show intermolecular interactions, leading to bathochromic shifts with respect to the spectra in solution. Cyclic voltammetry indicates that they have suitable HOMO and LUMO energy levels, and the oligomers containing thiophene segments show lower LUMO levels. The X-ray crystallography of compound

 Please wait while we load your content...
Please wait while we load your content...