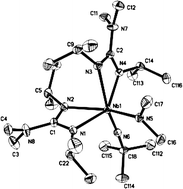
Novel mixed amido/imido/guanidinato complexes of niobium are reported. The complexes were synthesized by insertion of two equivalents of di-isopropylcarbodiimide (i-Pr-cdi) or bis-cyclohexylcarbodiimide (Cy-cdi) respectively, into the niobium–amido bonds of [Nb(NR2)3(N-t-Bu)] (1, R = Me; 2, R = Et) starting out from [NbCl3(N-t-Bu)(py)2] and the respective LiNR2 reagent (py = pyridine). Four representative examples of these mixed ligand amido/imido/guanidinato compounds were synthesized and were characterized by 1H-NMR, 13C-NMR, 15N-NMR, CHN-analysis, mass spectrometry and infra-red spectroscopy. The molecular structures of [Nb(NR2){η2-(i-Pr-N)2C(NR2)}2(N-t-Bu)] (3, R = Me; 4, R = Et) in the solid state were determined by single-crystal X-ray diffraction studies and are discussed together with the molecular structure of the starting compound [Nb(NMe2)3(N-t-Bu)] (1). The thermal properties of the new compounds depend on the substitution at the guanidinato ligand. Complexes of i-Pr-cdi are significantly more volatile than complexes of Cy-cdi as revealed by thermogravimetric analysis. Preliminary experiments using 3 as a single-molecule source for metal–organic chemical vapour deposition (MOCVD) in the absence of ammonia indicate the formation of the stoichiometric, and surprisingly carbon-free, cubic niobium nitride phase.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...