Design and total synthesis of unnatural analogues of the sub-nanomolar SERCA inhibitor thapsigargin†
Abstract
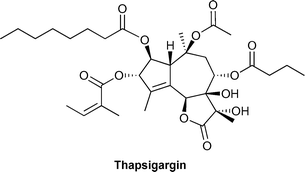
Thapsigargin is a densely oxygenated guaianolide which displays potent sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) binding affinities. The total syntheses of designed unnatural analogues of this important natural product are described. This article constitutes the chemical synthesis behind an ongoing project. Rational modifications have been made to the lactone region of thapsigargin in order to obtain derivatives for future structure–activity relationship studies.

 Please wait while we load your content...
Please wait while we load your content...