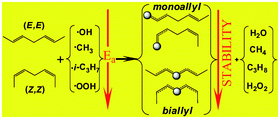
Unsaturated radicals, containing different number of delocalized electrons, are formed via H-atom abstractions with CH3, iso-C3H7, OOH and OH radicals from (Z,Z) and (E,E)-hepta-2,5-dienes. These reactions and the relative stability of the different allyl-type radicals formed, were studied within the BH&HLYP method, using a 6-311+G(3df,2p) basis set, as well as within the G3MP2 level of theory on BH&HLYP/6-31G(d) geometries. The biallyl type radicals (involving 5 electrons delocalized on 5 carbon atoms) are more stable, by about 47.6 ± 0.4 kJ mol−1, than monoallyl type radicals (which involve 3 electrons delocalized on 3 carbon atoms). Three types of the H-atom abstractions were distinguished: direct H-abstraction with CH3, indirect abstraction with a higher barrier height with iso-C3H7, OOH and a non-direct quasi-barrierless H-abstraction with OH radicals. These observations were also confirmed by the activation entropy versus activation enthalpy as well as the Evans–Polányi’s plots. The OOH-hepta-2,5-diene complexes are found to be extremely stable (from −19.6 to 22.3 kJ mol−1). The room temperature rate constants were calculated with transition state theory. Formations of monoallyl and biallyl radicals through H-abstraction with OH are fast; the calculated rate constants range from 5.84 × 10−11 to 1.92 × 10−9 cm3 molecule−1 s−1 at room temperature. These reactions may play a key role in the “very low temperature combustion” like biological oxidations.

 Please wait while we load your content...
Please wait while we load your content...