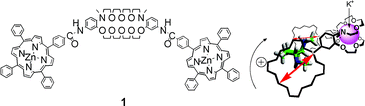
In our program for developing chirality manipulation systems, we synthesized bis(zinc porphyrin) 1, with a dibenzodiaza-30-crown-10 as a linker unit. Two structural features were examined. The aza-crown segment exhibited an intermolecular interaction with the zinc(II) of the porphyrin, capable of causing aggregation to form spherical nanostructures, as inferred by concentration-dependency of 1H NMR as well as scanning electron microscopy (SEM) observation. We also consider the crown-based conformation flexibility, in which accommodated K+ tunes the porphyrin orientation into the tweezers conformation, assisting chirality induction upon complexation with chiral diamine 2. The circular dichroism (CD) intensity change essentially reached a plateau at a [(1R,2R)-2] : [1] ratio of 2 : 1 for which a 45% enhancement in the amplitude of CD spectra was observed compared to the K+-free conditions. Use of the crown linker of 1 is not limited to promoting chirality induction with diamines in the presence of K+; chiroptical probing of unprotected amino acids (Lys, His, Trp, and Phe) using 1 was attained through liquid (1 in CH2Cl2)–liquid (the amino acids in 1 N KOH) two-phase extraction. The amphiphilic properties of the crown segment, as well as the K+-assisted tweezers conformation, make it possible to explore a potent way to develop chirality sensors for amino acids in water.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...