Biosynthesis of tetrapetalones
Abstract
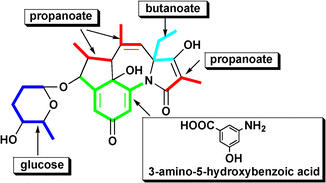
The biosynthesis of tetrapetalones (tetrapetalones A, B, C, and D) in Streptomyces sp. USF-4727 was studied by feeding experiments with [1-13C] sodium propanoate, [1-13C] sodium butanoate, [carbonyl-13C] 3-amino-5-hydroxybenzoic acid (AHBA) hydrochloride, and [1-13C] glucose, followed by analysis of the 13C-NMR spectra. These feeding experiments revealed that the four tetrapetalones were polyketide compounds constructed from propanoate, butanoate, AHBA, and glucose. The tetrapetalone biosynthetic pathway was also suggested in this study. In this pathway, tetrapetalone A (1) is synthesized by polyketide synthase (PKS) using AHBA as a starter unit, then the side chain of 1 is subjected to acetoxylation to produce tetrapetalone B (2). Additionally, 1 is oxidized and transformed into tetrapetalone C (3). In a similar way, 2 is converted to tetrapetalone D (4). Therefore, the biosynthetic relationship of the four tetrapetalones was indicated.

 Please wait while we load your content...
Please wait while we load your content...