Temperature dependence of the NO3 absorption cross-section above 298 K and determination of the equilibrium constant for NO3 + NO2 ↔ N2O5 at atmospherically relevant conditions†‡
Abstract
The reaction NO3 + NO2
↔ N2O5 was studied over the 278–323 K temperature range. Concentrations of NO3, N2O5, and NO2 were measured simultaneously in a 3-channel cavity ring-down spectrometer. Equilibrium constants were determined over atmospherically relevant concentration ranges of the three species in both synthetic samples in the laboratory and ambient air samples in the field. A fit to the laboratory data yielded 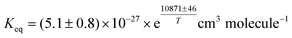 . The temperature dependence of the NO3 absorption cross-section at 662 nm was investigated over the 298–388 K temperature range. The line width was found to be independent of temperature, in agreement with previous results. New data for the peak cross section (662.2 nm, vacuum wavelength) were combined with previous measurements in the 200 K–298 K region. A least-squares fit to the combined data gave σ = [(4.582 ± 0.096) − (0.00796 ± 0.00031) ×
T] × 10−17 cm2 molecule−1.
. The temperature dependence of the NO3 absorption cross-section at 662 nm was investigated over the 298–388 K temperature range. The line width was found to be independent of temperature, in agreement with previous results. New data for the peak cross section (662.2 nm, vacuum wavelength) were combined with previous measurements in the 200 K–298 K region. A least-squares fit to the combined data gave σ = [(4.582 ± 0.096) − (0.00796 ± 0.00031) ×
T] × 10−17 cm2 molecule−1.


 Please wait while we load your content...
Please wait while we load your content...