Cross-coupling reactions of unprotected halopurine bases, nucleosides, nucleotides and nucleoside triphosphates with 4-boronophenylalanine in water. Synthesis of (purin-8-yl)- and (purin-6-yl)phenylalanines†
Abstract
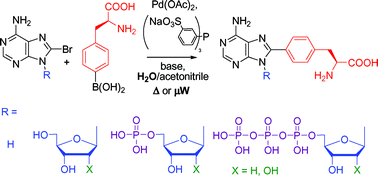
An expeditious and highly efficient single-step methodology for the introduction of a
- This article is part of the themed collection: Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

 Please wait while we load your content...
Please wait while we load your content...