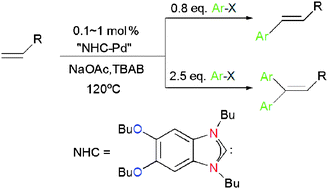
The Heck reactions of aryl bromides and chlorides are efficiently catalysed by palladium supported with benzimidazolylidenes generated in situ from N,N-dialkylbenzimidazolium salts in molten tetrabutylammonium bromide (TBAB) as ionic liquid reaction medium. Remarkable electronic effects from the benzimidazoliums on the catalysis have been observed. Reaction of 4-chloroacetophone with butyl acrylate catalysed by 1 mol% of the benzimidazolium–palladium catalyst systems containing 5,6-dibutoxy-N,N′-dibutylbenzimidazolium bromide 3, N,N′-dibutylbenzimidazolium bromide 1 or 5,6-difluoro-N,N′-dibutylbenzimidazolium bromide 4, gave 4-acetyl-trans-cinnamic acid ester in 93% (6 h), 79% (12 h) and 50% (12 h) yields, respectively, under otherwise identical conditions. For aryl bromides and activated aryl chlorides without steric hindrance, mono- and di-arylation of terminal olefins could be controllably effected, giving the corresponding di- and tri-substituted olefins in high yields. The electronic factors from aryl bromides are negligible in diarylation while electron-poor aryl halides react faster than the electron-rich ones in the monoarylation. Aryl halides with modest steric hindrance, such as 2,5-dimethylbromobenzene and 2-chlorobenzonitrile, react smoothly in the monoarylation of butyl acrylate with 0.1 and 1 mol% palladium loading, respectively. Mesityl bromide could also be converted to butyl mesityl-trans-cinnamate in 88% yield using an increased palladium loading of 1 mol%.

 Please wait while we load your content...
Please wait while we load your content...