1,1′-Bis(tert-butoxycarbonylamino)ferrocene (6), a protected derivative of 1,1′-diaminoferrocene, has been synthesized by a very convenient method and serves as a synthon for 1,1′-diaminoferrocene. Its structure in solid state and in solution has been studied by NMR and X-ray crystallography. 1,1′-Bis(tert-butoxycarbonylamino)ferrocene serves as starting material for the synthesis of amino acid conjugates of L- and D-alanine. The structures of these bioconjugates have been studied by NMR and CD spectroscopy and X-ray crystallography and reveal that the chiral organization of the podant amino acid chains is controlled by the chirality of the attached amino acid. The substituents engage in strong intramolecular H-bonding generating 14-membered H-bonded rings, a motif previously unrealized in ferrocene–amino acid and peptide conjugates.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
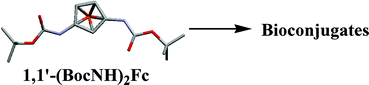

 Please wait while we load your content...
Please wait while we load your content...