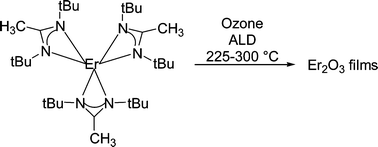
Treatment of anhydrous rare earth chlorides with three equivalents of lithium 1,3-di-tert-butylacetamidinate (prepared in situ from the di-tert-butylcarbodiimide and methyllithium) in tetrahydrofuran at ambient temperature afforded Ln(tBuNC(CH3)NtBu)3 (Ln = Y, La, Ce, Nd, Eu, Er, Lu) in 57–72% isolated yields. X-Ray crystal structures of these complexes demonstrated monomeric formulations with distorted octahedral geometry about the lanthanide(III) ions. These new complexes are thermally stable at >300 °C, and sublime without decomposition between 180–220 °C/0.05 Torr. The atomic layer deposition of Er2O3 films was demonstrated using Er(tBuNC(CH3)NtBu)3 and ozone with substrate temperatures between 225–300 °C. The growth rate increased linearly with substrate temperature from 0.37 Å per cycle at 225 °C to 0.55 Å per cycle at 300 °C. Substrate temperatures of >300 °C resulted in significant thickness gradients across the substrates, suggesting thermal decomposition of the precursor. The film growth rate increased slightly with an erbium precursor pulse length between 1.0 and 3.0 s, with growth rates of 0.39 and 0.51 Å per cycle, respectively. In a series of films deposited at 250 °C, the growth rates varied linearly with the number of deposition cycles. Time of flight elastic recoil analyses demonstrated slightly oxygen-rich Er2O3 films, with carbon, hydrogen and fluorine levels of 1.0–1.9, 1.7–1.9 and 0.3–1.3 atom%, respectively, at substrate temperatures of 250 and 300 °C. Infrared spectroscopy showed the presence of carbonate, suggesting that the carbon and slight excess of oxygen in the films are due to this species. The as-deposited films were amorphous below 300 °C, but showed reflections due to cubic Er2O3 at 300 °C. Atomic force microscopy showed a root mean square surface roughness of 0.3 and 2.8 nm for films deposited at 250 and 300 °C, respectively.

 Please wait while we load your content...
Please wait while we load your content...