Accurate ab initio determination of spectroscopic and thermochemical properties of mono- and dichlorocarbenes†
Abstract
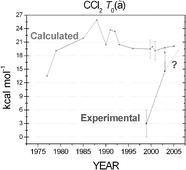
The best technically feasible values for the triplet–singlet energy gap and the enthalpies of formation of the HCCl and CCl2 radicals have been determined through the focal-point approach. The electronic structure computations were based on high-level coupled cluster (CC) methods, up to quadruple excitations (CCSDTQ), and large-size correlation-consistent basis sets, ranging from aug-cc-pVDZ to aug-cc-pV6Z, followed by extrapolation to the complete basis set limit. Small corrections due to core correlation, relativistic effects, diagonal Born–Oppenheimer correction, as well as harmonic and anharmonic zero-point vibrational energy corrections have been taken into account. The final estimates for the triplet–singlet energy gap, T0(ã), are 2170 ± 40 cm−1 for HCCl and 7045 ± 60 cm−1 for CCl2, favoring the singlet states in both cases. Complete quartic force fields in internal coordinates have been computed for both the ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) and ã states of both radicals at the frozen-core CCSD(T)/aug-cc-pVQZ level. Using these force fields vibrational energy levels of {HCCl, DCCl, CCl2} up to {6000, 5000, 7000} cm−1 were calculated both by second-order vibrational perturbation theory (VPT2) and variationally. These results, especially the variational ones, show excellent agreement with the experimentally determined energy levels. The enthalpies of formation of HCCl (
and ã states of both radicals at the frozen-core CCSD(T)/aug-cc-pVQZ level. Using these force fields vibrational energy levels of {HCCl, DCCl, CCl2} up to {6000, 5000, 7000} cm−1 were calculated both by second-order vibrational perturbation theory (VPT2) and variationally. These results, especially the variational ones, show excellent agreement with the experimentally determined energy levels. The enthalpies of formation of HCCl (![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1A′) and CCl2(
1A′) and CCl2(![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) 1A1), at 0 K, are 76.28 ± 0.20 and 54.54 ± 0.20 kcal mol−1, respectively.
1A1), at 0 K, are 76.28 ± 0.20 and 54.54 ± 0.20 kcal mol−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...