We have studied the mobilities of calcium and sodium ions in silicate glasses of compositions xNa2O·(3 −
x)CaO·4SiO2 with x
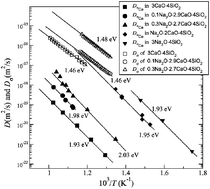
= 0.0, 0.1, 0.3, 1.0 and 3.0 by means of radiotracer diffusion, electrical conductivity measurements, and dynamic mechanical thermal analyses. In glasses containing sodium oxide, the Na+ ions are much more mobile than the Ca2+ ions, and are, therefore, governing the electrical conductivity. In the pure calcium silicate glass, the activation energy of Ca2+ diffusion is higher than the activation energy of the electrical conductivity. This provides strong evidence that the electrical conductivity of this glass is not determined by the migration of Ca2+ ions, but by impurity charge carriers, which are most likely Na+ ions. We sketch the composition-dependent mobilities of Na+ and Ca2+ ions in soda-lime silicate glasses with variable Na2O and CaO content. Our results indicate that the coordination environment of Ca2+ ions remains unchanged when CaO is replaced by Na2O which is consistent with recent results of molecular dynamic simulations. Moreover, our results confirm the formation of dissimilar Na–Ca pairs which lead to a non-random mixing of the cations in the glass. The formation of such pairs was recently deduced from nuclear magnetic resonance spectra of soda-lime silicate glasses.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...