Three polymeric cobalt(II) complexes of formulae [Co(pyo)2(dca)2]n
(1), [Co3(ac)4(bpe)3
(dca)2]n
(2) and [{Co(male)(H2O)2(H2O)]n
(3) [pyo, pyridine-N-oxide; dca, dicyanamide; ac, acetate; bpe, 1,2-bis-(4-pyridyl)ethane and male, maleate] have been synthesized and characterized structurally as well as magnetically. The structure determination of complex 1 shows that each octahedral Co(II) in the 1D coordination chain is attached with four μ-1,5-dicyanamide and two pendant pyridine-N-oxide ligands, which form mutual relationships with other 1D chains through non-covalent π-π interactions, giving rise to a 2D infinite sheet-like structure. The molecular structure reveals that complex 2 adopts an infinite three-leg ladder-like structure in which three parallel 1D Co(bpe) chains are connected by syn-syn and oxo bridging acetate ligands. The dicyanamide ligands are pendant to the terminal Co(II) centers. Complex 3 is an infinite 3D network in which carboxylate groups of the maleate ligand are linked to Co(II) centers in syn-anti fashion. The structure of complex 3 has already been reported. The variable temperature (300–2 K) magnetic measurements have been performed for all three complexes. In the case of 2, the full structure can be rationalized as quasi-isolated trimers; the exchange Hamiltonian that describes magnetic interactions between the effective S′
= 1/2 spins, at low temperature is 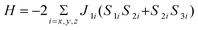 . Fixing g⊥
= 6.01 and g∣∣
= 2.25, according to the EPR measurements at 4 K, 2J
=
−3.3 cm−1 is the best-fit parameter. For 1 and 3, complete fits are not possible for calculating the corresponding J parameters for the one- and three-dimensional structures, respectively. Only an approximate J value has been calculated for 1.
. Fixing g⊥
= 6.01 and g∣∣
= 2.25, according to the EPR measurements at 4 K, 2J
=
−3.3 cm−1 is the best-fit parameter. For 1 and 3, complete fits are not possible for calculating the corresponding J parameters for the one- and three-dimensional structures, respectively. Only an approximate J value has been calculated for 1.
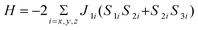 . Fixing g⊥
= 6.01 and g∣∣
= 2.25, according to the
. Fixing g⊥
= 6.01 and g∣∣
= 2.25, according to the ![Graphical abstract: Synthesis, crystal structure and magnetic behavior of three polynuclear complexes: [Co(pyo)2(dca)2]n, [Co3(ac)4(bpe)3(dca)2]n and [{Co(male)(H2O)2}(H2O)]n [pyo, pyridine-N-oxide; dca, dicyanamide; ac, acetate; bpe, 1,2-bis-(4-pyridyl)ethane and male, maleate]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B401928H&imageInfo.ImageIdentifier.Year=2004)

 Please wait while we load your content...
Please wait while we load your content...