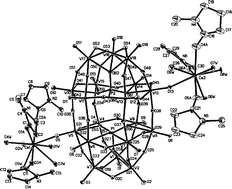
Three polyoxometalate-based composite compounds containing zero-dimensional cross-like and one-dimensional chain-like structures, [Gd(DMF)6(H2O)2]2·[P2W18O62]·4DMF·2H2O (1), [Ce(NMP)3(H2O)5][Ce(NMP)3(H2O)4][(P2W18O62)]·4H2O (2) and [{Gd(DMF)6}{Gd(DMF)7}(P2W18O62)]·0.5DMF (3), were constructed from octadecatungstodiphosphorate clusters interacting with [RE(donor)]3+ [RE = Gd(III), Ce(III); donor = DMF (N,N-dimethylformamide), NMP (N-methyl-2-pyrrolidone)] groups. Single crystal X-ray structural analyses reveal that, for compound 1, the interactions between the polyanions and the charge compensating cations are mainly hydrogen bonds, van der Waals forces and electrostatic forces; for compound 2, two terminal oxygen (Ot) atoms located in “belt” sites of the polyanion are coordinated by RE cations, which generates an unprecedented zero-dimensional isolated structure; for compound 3, the adjacent polyanions are polymerized to form a one-dimensional infinite linear chain by the bridging RE coordination cations and exhibit the type –ACACAC–. The apparent difference in the ESR spectra of the two compounds containing Gd(III) ions seems to be related to different coordination environments of the Gd(III) ions. Thermal analyses show that the frameworks of the polyanions decompose at 611, 615 and 602 °C in compounds 1–3, respectively, and the stability of polyanions in compounds 1–3 is much stronger than that in the parent acid.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...