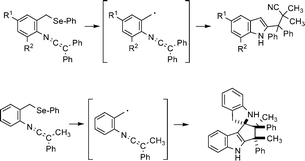
The intramolecular addition of benzylic radicals, generated from benzyl phenyl selenides by the action of tris(trimethylsilyl)silane and AIBN or AIBMe, onto neighbouring ketenimine functions is studied. The persistent α-(indol-2-yl)benzyl radicals, resulting from such cyclization processes, undergo cross-coupling with the tert-alkyl radicals arising from the thermal decomposition of the AIBN or AIBMe initiators to give, respectively, 3-(1H-indol-2-yl)propiononitriles 10 or propanoates 14. A rare spiropentacyclic compound 17 containing two indole fragments also resulted from one of these radical reactions. The crystal and molecular structures of 10d and 17 have been solved by X-ray analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...