We investigated Li+/H+ exchange in the lithium ion conductors (LISICONS) [Li2+2xZn1−xGeO4; x
= 0.5 (I) and x
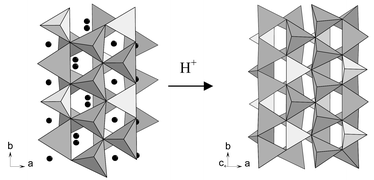
= 0.75 (II)] and their parent, γ-Li2ZnGeO4. Facile exchange of approximately 2x lithium ions per formula unit occurs with both the LISICONS in dilute acetic acid, while the parent material does not exhibit an obvious Li+/H+ exchange under the same conditions. The results can be understood in terms of lithium ion distribution in the crystal structures: the parent Li2ZnGeO4, where all the lithium ions form part of the tetrahedral framework structure, does not exhibit a ready Li+/H+ exchange; LISICONS, where lithium ions are distributed between framework (tetrahedral) and nonframework sites, undergo a facile Li+/H+ exchange of the nonframework site lithium ions. Accordingly, Li+/H+ exchange in dilute aqueous acetic acid provides a convenient probe to distinguish between the mobile and the immobile lithium ions in lithium ion conductors.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...