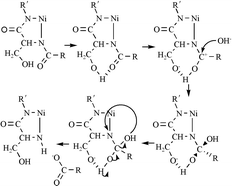
The coordination properties of Ni(II) ions towards the terminally blocked (CH3CONH- and -CONH2) hexapeptides -TESHHK-, -TASHHK-, -TEAHHK-, -TESAHK- and -TESHAK- were studied by using potentiometric and spectroscopic techniques (UV/Vis, CD, NMR). The peptides were chosen in such a way as to compare the effect of Glu, Ser and His residues on the stability, the coordination and hydrolytic abilities of the complexes formed. All peptides bind to Ni(II) ions initially through one or two imidazole nitrogens in weakly acidic and neutral solutions forming slightly distorted octahedral complexes. At higher pH values, a series of square-planar complexes are formed, where Ni(II) ions bind simultaneously through an imidazole and three amide nitrogens in an equatorial plane. This proposed conformation includes the participation of only one imidazole nitrogen, in the case of all peptides, in the coordination sphere of Ni(II) ions. In basic solutions, the peptides -TASHHK- and -TESAHK- were hydrolyzed in a Ni(II)-assisted fashion. No hydrolytic processes were noticed in peptides -TEAHHK- and -TESHAK- where the Ser or His-5 residues are replaced with the Ala residue. The Ni(II)-assisted hydrolysis of the analogues of -TESHHK- may provide an insight into the novel mechanism of genotoxicity, combining the damage to the nucleosome with the generation of further toxic Ni(II) species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...