A dual-mode rechargeable lithium–bromine/oxygen fuel cell
Abstract
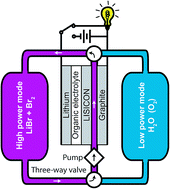
In order to meet the versatile power requirements of autonomous underwater vehicles (AUV), we propose a rechargeable lithium–bromine/seawater fuel cell with a protected lithium metal anode to provide high specific energy at either low-power mode with seawater (oxygen) or high-power mode with bromine catholytes. The proof-of-concept fuel cell with a flat catalyst-free graphite electrode can discharge at 3 mW cm−2 with seawater, and 9 mW cm−2 with dilute bromine catholytes. The fuel cell can also be recharged with LiBr catholytes efficiently to recover the lithium metal anode. Scanning electron microscopy images reveal that both the organic electrolyte and the bromine electrolyte corrode the solid electrolyte plate quickly, leading to nanoporous pathways that can percolate through the plate, thus limiting the cell performance and lifetime. With improved solid electrolytes or membraneless flow designs, the dual-mode lithium–bromine/oxygen system could enable not only AUV but also land-based electric vehicles, by providing a critical high-power mode to high-energy-density (but otherwise low-power) lithium–air batteries.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...