Particle-size and morphology dependence of the preferred interface orientation in LiFePO4 nano-particles
Abstract
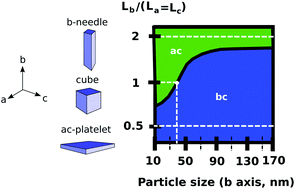
We gain new insights into the equilibrium properties and potential two-phase lithiation mechanisms in LiFePO4 nano-particles by conducting a first-principles investigation of the anisotropic chemical interfacial energy landscape in LiFePO4. The chemical interfacial energy per unit area along the ac plane is found to be remarkably low (7 mJ m−2) with respect to the bc (115 mJ m−2) and ab (95 mJ m−2) chemical interfacial energies. Because chemical interfacial energy and coherency strain energy have different anisotropies, the thermodynamically stable interface orientation is shown to depend both on the particle size and on the particle morphology. In particular, ac interfaces are favored for isotropic particles below 40 nm. This indicates that, if experimentally-relevant nano-particles were to (de)lithiate under a thermodynamic two-phase mechanism, the resulting front would be orientated along the ac plane, and not along the bc plane as is assumed in most lithiation models in the literature.

 Please wait while we load your content...
Please wait while we load your content...