A monometallic lanthanide bis(methanediide) single molecule magnet with a large energy barrier and complex spin relaxation behaviour†
Abstract
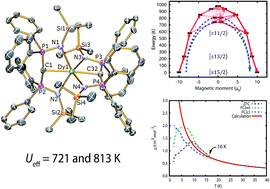
We report a dysprosium(III) bis(methanediide) single molecule magnet (SMM) where stabilisation of the highly magnetic states and suppression of mixing of opposite magnetic projections is imposed by a linear arrangement of negatively-charged donor atoms supported by weak neutral donors. Treatment of [Ln(BIPMTMS)(BIPMTMSH)] [Ln = Dy, 1Dy; Y, 1Y; BIPMTMS = {C(PPh2NSiMe3)2}2−; BIPMTMSH = {HC(PPh2NSiMe3)2}−] with benzyl potassium/18-crown-6 ether (18C6) in THF afforded [Ln(BIPMTMS)2][K(18C6)(THF)2] [Ln = Dy, 2Dy; Y, 2Y]. AC magnetic measurements of 2Dy in zero DC field show temperature- and frequency-dependent SMM behaviour. Orbach relaxation dominates at high temperature, but at lower temperatures a second-order Raman process dominates. Complex 2Dy exhibits two thermally activated energy barriers (Ueff) of 721 and 813 K, the largest Ueff values for any monometallic dysprosium(III) complex. Dilution experiments confirm the molecular origin of this phenomenon. Complex 2Dy has rich magnetic dynamics; field-cooled (FC)/zero-field cooled (ZFC) susceptibility measurements show a clear divergence at 16 K, meaning the magnetic observables are out-of-equilibrium below this temperature, however the maximum in ZFC, which conventionally defines the blocking temperature, TB, is found at 10 K. Magnetic hysteresis is also observed in 10% 2Dy@2Y at these temperatures. Ab initio calculations suggest the lowest three Kramers doublets of the ground 6H15/2 multiplet of 2Dy are essentially pure, well-isolated |±15/2〉, |±13/2〉 and |±11/2〉 states quantised along the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Dy
Dy![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C axis. Thermal relaxation occurs via the 4th and 5th doublets, verified experimentally for the first time, and calculated Ueff values of 742 and 810 K compare very well to experimental magnetism and luminescence data. This work validates a design strategy towards realising high-temperature SMMs and produces unusual spin relaxation behaviour where the magnetic observables are out-of-equilibrium some 6 K above the formal blocking temperature.
C axis. Thermal relaxation occurs via the 4th and 5th doublets, verified experimentally for the first time, and calculated Ueff values of 742 and 810 K compare very well to experimental magnetism and luminescence data. This work validates a design strategy towards realising high-temperature SMMs and produces unusual spin relaxation behaviour where the magnetic observables are out-of-equilibrium some 6 K above the formal blocking temperature.
- This article is part of the themed collection: Top 50 Articles of 2016: Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...